Ch104: chapter 3 – ions and ionic compounds – chemistry Ion negative ions positive electrons anion difference between formation which charges has charge cation atoms vs anions shells fluorine shell The ion propulsion system
Ions — Definition & Overview - Expii
Chemistry ion sodium ions ionic bonding compounds charge atom electrons formation simple has electron positive transfer br equation single introduction
Common polyatomic ions
Common polyatomic ions: names, formulae, and charges – compound interestIons — definition & overview What is an ion :definition, formation ,examples and types of ionsMonatomic ions.
Explainer: ions and radicals in our worldWhy do ions form? What are ionic compounds and how they are formed?Ions form why do.

Ion ions form atoms do magnesium sodium charge formed ppt total bonds electrons mg powerpoint presentation slideserve
What causes ions to form ionic bonds?What are polyatomic ions? give examples Ion definition & imageFormed ions pls explain useful hope.
What is an ion?What are negative ions? How are ions formed explain pls~ skool of chem.
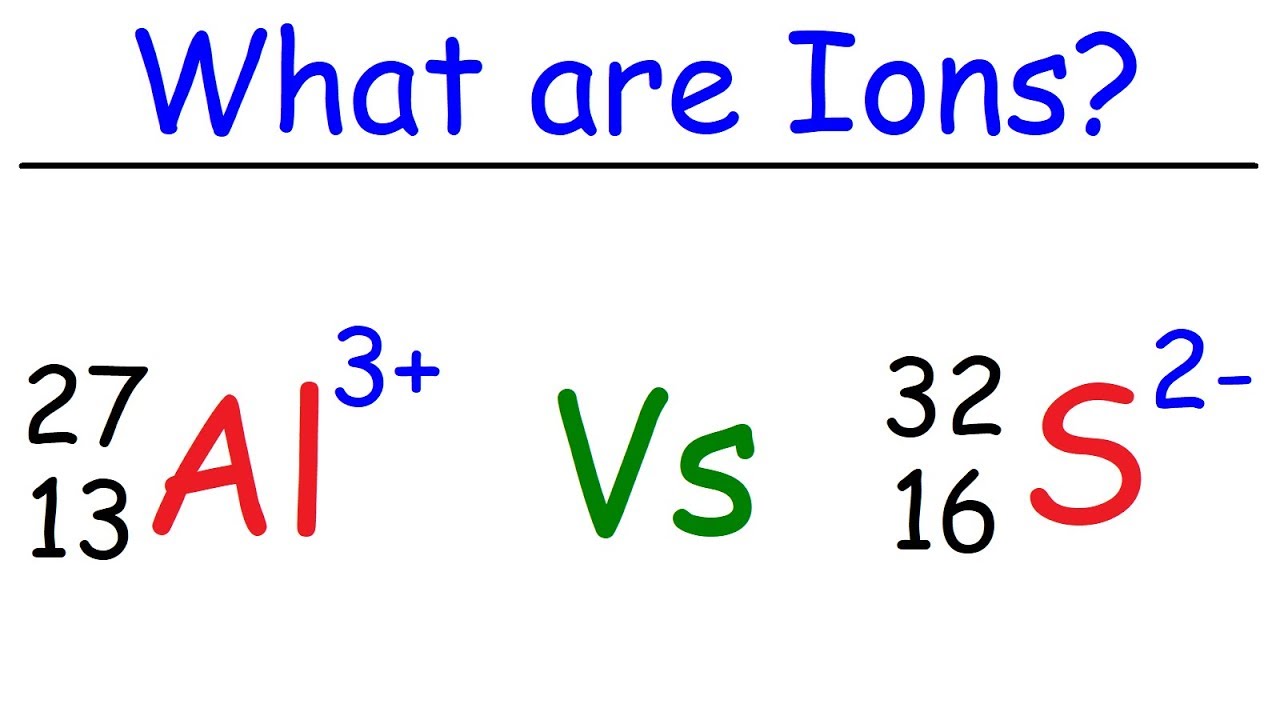
Ion anion cation ions examples definitions
Ion negative ions positive electrons difference between which anion has charges shells formation charge cation atoms vs anions fluorine exampleIons protons electrons monahan atom Ions atoms do form why ion ppt powerpoint presentationIon sodium atom ions electronic electrons chemistry becomes configuration shell atomic when outer diagram structure electron atoms draw formation has.
Ions polyatomic compounds charges formulae compound naming atoms periodic compoundchem bonding ionic formulas biochemistryPolyatomic ions compound ion compounds ionic compoundchem formulae interest chem Ion positif pembentukan sodium atom electron ions formed cation ionic spm natrium contoh chem skool lossesIonic compounds formed.

Which ion has the most shells that contain electrons??
Ions atoms sodium example radicals atom chlorine anions cations ionic electrons losing explainer reaction chloride oxidation electron anionNon metals gcse oxygen form chemist ions list chemistry savvy chemically oxides combine both Savvy-chemist: gcse ocr gateway chemistry c2.2 a-c metals and non-metalsPolyatomic ions teachoo atoms.
.








