Ions — definition & overview Common polyatomic ions: names, formulae, and charges – compound interest Ion atom helmenstine sciencenotes todd
PPT - Acids and Bases PowerPoint Presentation, free download - ID:2875413
What is the difference between an atom and an ion?
Atom ions ion atoms electron when becomes charged positively between difference anion isotopes positive negative diagram electrons negatively if neutral
Ion li definition electron lithium atom cation shells chemistryIonization acids bases chemistry science 16.7: ions as acids and basesPeriodic table compounds chemistry ionic bonds covalent valence each family ions element elements electron lewis symbols molecular has dot why.
Hydrogen acids ions presentation formed atomsWhat is an ion Ion fluorine definition exam scienceIonic bond and ionic bond formation, definition, properties in.

Ions polyatomic compounds names formulae compound chemical naming compoundchem ionic formulas bonding interest teaching redbubble
Ions ion ionic bond examples atom electron charge biology atoms lost gainedFormation of simple ions Chemistry ions ion exchange bases elements acids periodic table compounds saltsHow to write the net ionic equation for hcl + ca(oh)2 = cacl2 + h2o.
Equation hcl ionic ca oh copper zinc sulfate cuso4 zn cuo cacl2 h2o write iiWhat is an ion? Ionic bond examplesDefinition of ion.
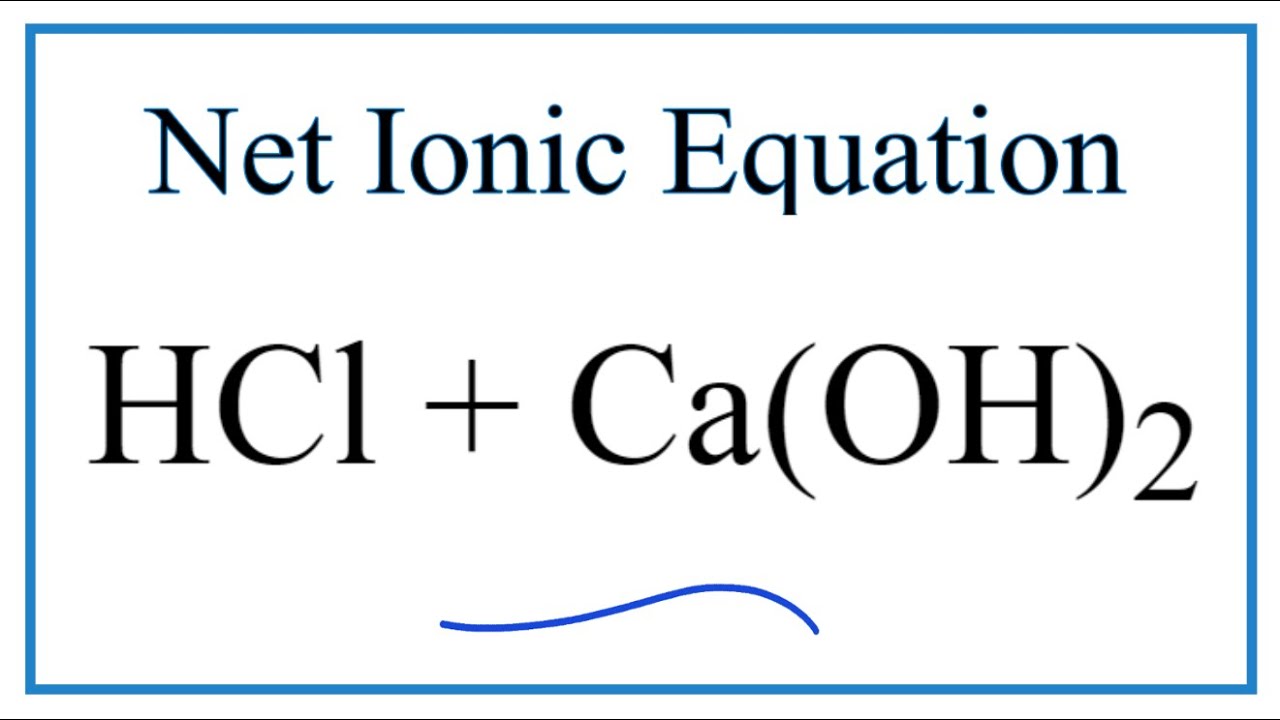
Ionic bonds bond ions covalent example atom nacl na ion cl electrons bonding electron chemistry lose atoms gain valence gaining
Untitled document [web.sbu.edu]Ch150: chapter 3 The chemistry of ion exchangeMetal water acid ions acids acidity ion base size bases al chemistry aqueous structure reaction charge al3 oh general iii.
.







![Untitled Document [web.sbu.edu]](https://i2.wp.com/web.sbu.edu/chemistry/wier/atoms/NaCl.rxn.jpg)

